The man who has ceased to learn ought not to be allowed
to wander around loose in these dangerous days.
—M. M. Coady
A. Who Is the Intended Audience?
This book was written with today’s students in mind. It provides instantaneous access to information; does not waste time on extraneous details; cuts right to the point; uses more bullets to make information easier to access; and includes new, novel problems on chemical reaction engineering (e.g., solar energy).1 The interaction between the text and Web site (http://www.umich.edu/~elements/6e/) breaks new ground and provides one of the most comprehensive active learning resources available. With the advent of sliders in both Wolfram and Python, students can explore the reactions and the reactor in which they occur, by carrying out simulation experiments and then writing a set of conclusions to describe what they found.
1 This Introduction is a condensed version of the full Preface/Introduction found on the Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf).
This book and interactive Web site are intended for use as both an undergraduate-level and a graduate-level text in chemical reaction engineering. The undergraduate course/courses usually focus on Chapters 1–13; the graduate course material includes topics such as diffusion limitations, effectiveness factors (discussed in Chapters 14 and 15), nonideal reactors, and residence time distribution (discussed in Chapters 16–18) along with the additional material and Professional Reference Shelf (PRS) on the Web site.
This edition emphasizes chemical reactor safety by ending each chapter with a safety lesson called And Now… A Word From Our Sponsor-Safety (AWFOS–S). These lessons can also be found on the Web site at http://umich.edu/~safeche/.
B. What Are the Goals of This Book?
B.1 To Have Fun Learning Chemical Reaction Engineering (CRE)
Chemical reaction engineering (CRE) is a great subject that is fun to learn and is the heart of chemical engineering. I have tried to provide a little Michigan humor as we go. Take a look at the humorous YouTube videos (e.g., “Black Widow” or “Chemical Engineering Gone Wrong”) that illustrate certain principles in the text. These videos were made by chemical engineering students at the universities of Alabama and Michigan. In addition, I have found that students enjoy the Interactive Computer Games (ICGs) that, along with the videos, are linked from the CRE homepage (http://www.umich.edu/~elements/6e/index.html).
B.2 To Develop a Fundamental Understanding of Reaction Engineering
The second goal of this book is to help the reader clearly understand the fundamentals of CRE. This goal is achieved by presenting a structure that allows the reader to solve reaction engineering problems through reasoning rather than through memorization and recall of numerous equations and the restrictions and conditions under which each equation applies (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf.
B.3 To Enhance Thinking Skills
A third goal of this text is to enhance critical thinking skills and creative thinking skills. How does the book help enhance your critical and creative thinking skills? We discuss ways to achieve this enhancement in Table P-2, Critical Thinking Questions; Table P-3, Critical Thinking Actions; and Table P-4, Practicing Creative Thinking, in the complete preface on the CRE Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf) and also from the Problem Solving Web site (http://umich.edu/~scps/).
C. What Is the Structure of CRE?
C.1 What Are the Concepts That Form the Foundation of CRE?
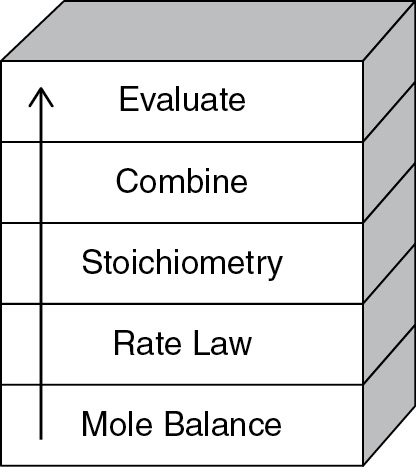
The strategy behind the presentation of material is to build continually on a few basic ideas in CRE to solve a wide variety of problems. The building blocks of CRE and the primary algorithm allow us to solve isothermal CRE problems through logic rather than memorization. We start with the Mole Balance Building Block (Chapter 1) and then place the other blocks one at a time on top of the others until we reach the Evaluate Block (Chapter 5), by which time we can solve a multitude of isothermal CRE problems. As we study each block, we need to make sure we understand everything in that block and be sure not to cut corners by leaving anything out so we don’t wind up with a stack of cylindrical blocks. An animation of what happens to such a stack is shown at the end of Lecture 1 notes (http://www.umich.edu/%7Eelements/6e/lectures/umich.html).
For nonisothermal reactions, we replace the “Combine” building block in Figure I-1 with the “Energy Balance” building block because nonisothermal reactions almost always require a computer-generated solution. Consequently, we don’t need the “Combine” block because the computer combines everything for us. From these pillars and building blocks, we construct our CRE algorithm:
Mole Balance + Rate Laws + Stoichiometry + Energy Balance + Combine → Solution
C.2 What Is the Sequence of Topics in Which This Book Can Be Used?
The selection and order of topics and chapters are shown in Figure P-3 in the Complete Preface/Introduction on the Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf). There are notes in the margins, which are meant to serve two purposes. First, they act as guides or commentary as one reads through the material. Second, they identify key equations and relationships that are used to solve CRE problems.
Margin Notes
D. What Are the Components of the CRE Web Site?
The interactive companion Web site material has been significantly updated and is a novel, and integral part of this book. The main purposes of the Web site are to serve as an interactive part of the text with enrichment resources. The home page for the CRE Web site (http://www.umich.edu/~elements/6e/index.html) is shown in Figure I-2. For discussion of how to use the Web site and text interactively, see Appendix I.

(http://www.umich.edu/~elements/6e/index.html).
The objectives of the Web site are fourfold:
- To facilitate the interactive learning of CRE by using the companion Web site and Wolfram and Python sliders to explore Living Example Problems to gain a deep understanding of the reaction and the reactors in which they take place.
- To provide additional technical material in the extended material and in the Professional Reference Shelf.
- To provide tutorial information and self-assessment exercises such as the i>clicker questions.
- To make the learning of CRE fun through the use of interactive games, LEP simulations, and computer experiments, which allow one to use Inquiry-Based Learning (IBL) to explore the concepts of CRE.
D.1 How to Use the Web Site
I would like to expand a bit on a couple of things that we use extensively, namely the useful links. These items can be accessed by clicking on the Chapter number on the Home Page. After clicking on Chapter 1 shown in Figure I-3, one will arrive at
(http://www.umich.edu/~elements/6e/01chap/obj.html#/).
The important point I want to make here is the list of all resources shown in Figures I-3 and I-4. In addition to listing the objectives for this chapter, you will find all the major hot buttons, such as
The Living Example Problems (LEPs), including COMSOL, have all numerical Example Problems programmed and ready for use with the click of a button. The Extra Help includes interactive notes, screen casts, and techniques that facilitate learning and studying. The Additional Material and Professional Reference Shelf provide expanded derivations and material that is relevant to CRE, but did not make the final cut owing to limitations of the thickness of the book; that is, students can’t concentrate about CRE if their backpacks are so heavy they are suffering from carrying them. The Self Tests and i>Clicker Questions help readers gauge their level of understanding.
D.2 Living Example Problems (LEPs)
What are LEPs? LEPs are Living Example Problems that are really simulations that can be used to carry out experiments on the reactor and the reactions occurring inside the reactor. Here, rather than being stuck with the parameter values the author gives, the LEPs allow you to change the value of a parameter and see its effect on the reactor’s operation. LEPs have been unique to this book since their invention and inclusion in the Third Edition of this title, published in 1999. However, Wolfram and Python have allowed us to take LEPs to a new level, resulting in a minor paradigm shift. The LEPs use simulation software, which can be downloaded directly onto one’s own computer in order to “play with” the key variables and assumptions. Using the LEPs to explore the problem and asking “What if…?” questions provide students with the opportunity to practice critical and creative thinking skills. To guide students in using these simulations, questions for each chapter are given on the Web site (e.g., http://www.umich.edu/~elements/6e/12chap/obj.html).2 In this edition, there are more than 80 interactive simulations (LEPs) provided on the Web site. It is the author’s strong belief that using the LEP sliders will develop an intuitive feel for Chemical Reaction Engineering (CRE).
Leave a Reply