Be sure to view the actual photographs of industrial reactors on the CRE Web site so you will know them when you run into them. There are also links to view reactors on different Web sites. The CRE Web site also includes a portion of the Visual Encyclopedia of Equipment, encyclopedia.che.engin.umich.edu, “Chemical Reactors” developed by Dr. Susan Montgomery and her students at the University of Michigan. Also see Professional Reference Shelf on the CRE Web site for “Reactors for Liquid-Phase and Gas-Phase Reactions,” along with photos of industrial reactors, and Expanded Material on the CRE Web site.2
2 Chem. Eng., 63(10), 211 (1956). See also AIChE Modular Instruction Series E, 5 (1984).

In this chapter, and on the CRE Web site, we’ve introduced each of the major types of industrial reactors: batch, stirred tank, tubular, and fixed bed (packed bed). Many variations and modifications of these commercial reactors (e.g., semibatch, fluidized bed) are in current use and these reactors will be discussed in Chapters 6 and 10, respectively. For further elaboration, refer to the detailed discussion of industrial reactors given by Walas.3
3 S. M. Walas, Reaction Kinetics for Chemical Engineers, New York: McGraw-Hill, 1959, Chap. 11.
http://encyclopedia.che.engin.umich.edu/Pages/Reactors/menu.html
The CRE Web site describes industrial reactors, along with typical feed and operating conditions. In addition, two solved example problems for Chapter 1 can be found on the CRE Web site, http://www.umich.edu/~elements/6e.
1.6 And Now… A Word from Our Sponsor–Safety 1 (AWFOS–S1 Safety)
A note to students: In this sixth edition of Elements of Chemical Reaction Engineering, I am including a section at the end of each chapter to bring a greater awareness to process safety. A critical aspect of process safety is “anticipating” what could go wrong in a chemical process and ensuring it won’t go wrong. Equipment and processes involving exothermic chemical reactions are some of the most at risk in a chemical plant. Consequently, each chapter will end with a segment “And Now… A Word From Our Sponsor–Safety” (AWFOS–S). In addition, to highlight process safety across the chemical engineering curriculum, a Web site (http://umich.edu/~safeche/) has been developed that features a safety module specific to every core chemical engineering lecture course plus lab safety. In this chapter, we define process safety along with a very brief discussion on why it is important to study process safety.
1.6.1 What Is Chemical Process Safety?
Chemical process safety is a blend of engineering and management practices focused on preventing accidents, namely explosions, fires, and toxic releases that result in loss of life and property.
1.6.2 Why Study Process Safety?

Industrial disasters such as UCIL Bhopal, T2 Laboratories, BP Texas City, and Flixborough have collectively killed and injured thousands of people and caused billions of dollars in damage to chemical plants and nearby communities. Accidents such as these occur because chemical engineering processes are some of the most potentially dangerous due to extreme operating conditions and the use of explosive, reactive, and flammable materials. What surprises people is that most of these chemical engineering accidents, such as those listed in the Chemical Safety Board Videos on the companion Web site (http://umich.edu/~safeche/) were preventable. They were the result of poor engineering decisions, made by people who lacked fundamental understanding of basic chemical engineering concepts and chemical engineering safety. Thus, knowing the fundamentals of chemical engineering and process safety may save your life and the lives of innocent people, and prevent the loss of millions of dollars of material and equipment.
Engineers have an ethical and professional obligation to work only in areas for which they are competent and qualified. The best way to prevent future industrial disasters is to understand how to effectively and safely design, operate, and troubleshoot chemical processes. To prepare a prevention plan, we must take the time and effort to understand chemical processes and chemical process safety. To help achieve this understanding, the last section of every chapter has a tutorial, AWFOS–S, that can help you prevent accidents.
A comparison of process safety and personal safety is very succinctly given on the Web site (http://www.energysafetycanada.com/files/pdf/Personal_vs_Process_Safety_v3.pdf).
Closure. The goal of this text is to weave the fundamentals of chemical reaction engineering into a structure or algorithm that is easy to use and apply to a variety of problems. We have just finished the first building block of this algorithm: mole balances.

This algorithm and its corresponding building blocks will be developed and discussed in the following chapters:
- Mole Balance, Chapters 1 and 2
- Rate Law, Chapter 3
- Stoichiometry, Chapter 4
- Isothermal Reactor Design, Chapter 5CombineEvaluate
- Energy Balance, Chapters 11–13
With this algorithm, one can approach and solve chemical reaction engineering problems through logic rather than memorization.
A Word of Caution: The falling CRE Tower. As we proceed through the next five chapters, we will see how these building blocks form a tower. Now, if one cuts corners when studying this material, the building blocks become cylinders and as a result the tower becomes unstable and all of the understanding of CRE falls apart. See http://www.umich.edu/~elements/6e/01chap/assets/player/KeynoteDHTMLPlayer.html#3.
Summary
Each chapter summary gives the key points of the chapter that need to be remembered and carried into succeeding chapters.
- A mole balance on species j, which enters, leaves, reacts, and accumulates in a system volume V, isIf, and only if, the contents of the reactor are well mixed will the mole balance (Equation (S1-1)) on species A give
- The kinetic rate law for rj is
- The rate of formation of species j per unit volume (e.g., mol/s·dm3)
- Solely a function of the properties of reacting materials and reaction conditions (e.g., concentration [activities], temperature, pressure, catalyst, or solvent [if any]) and does not depend on reactor type
- An intensive quantity (i.e., it does not depend on the total amount)
- An algebraic equation, not a differential equation (e.g., −rA = kCA or )
- Mole balances on species A in four common reactors are shown in Table S1-1.TABLE S1-1 SUMMARY OF REACTOR MOLE BALANCES ReactorCommentMole Balance Differential FormAlgebraic FormIntegral Form
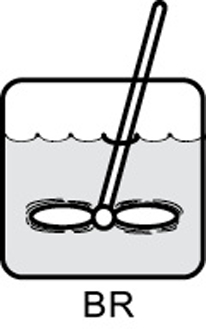 BRNo spatial variations
BRNo spatial variations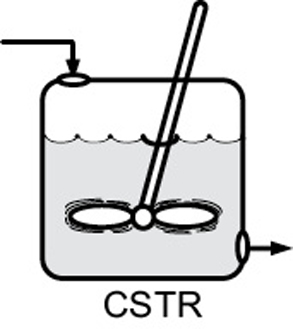 CSTRNo spatial variations, steady state——
CSTRNo spatial variations, steady state——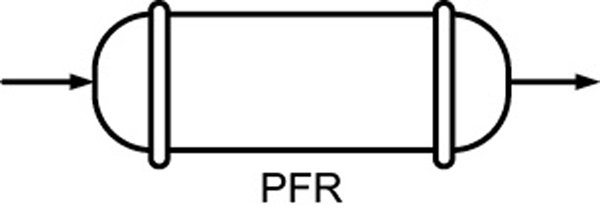 PFRSteady state
PFRSteady state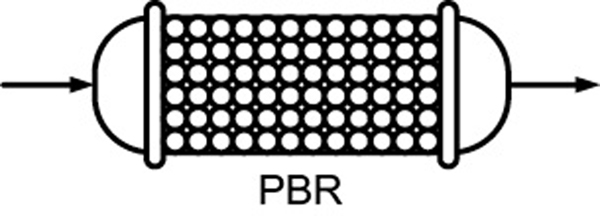 PBRSteady state
PBRSteady state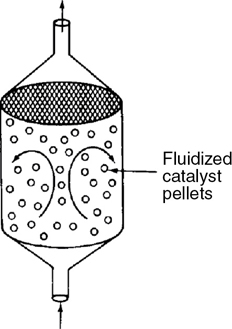 Fluidized CSTRSteady state——
Fluidized CSTRSteady state——
CRE Web Site Materials
(http://www.umich.edu/~elements/6e/01chap/obj.html#/)
Getting Unstuck on a Problem

(http://www.umich.edu/~elements/6e/01chap/iclicker_ch1_q1.html)
Smog in L.A. Web Module

Photograph by Radoslaw Lecyk/Shutterstock
(http://www.umich.edu/~elements/6e/web_mod/la_basin/index.htm)
Living Example Problem:
http://www.umich.edu/~elements/6e/01chap/live.html
Interactive Computer Games (http://www.umich.edu/~elements/6e/icm/index.html)
A. Quiz Show I (http://www.umich.edu/~elements/6e/icm/kinchal1.html)
This game could help prepare you for the AIChE student chapter Jeopardy Competition held each year at the Annual AIChE meeting.
Questions, Simulations, and Problems
I wish I had an answer for that, because I’m getting tired of answering that question.
—Yogi Berra, New York Yankees
Sports Illustrated, June 11, 1984
The subscript to each of the problem numbers indicates the level of difficulty, that is, A, least difficult; B, moderate difficulty; C, fairly difficult; D, (double black diamond), most difficult. A = • B = ▪ C = ♦ D = ♦♦ For example, P1-5B means “1” is the Chapter number, “5” is the problem number, “B” is the problem difficulty, in this case B means moderate difficulty.
Before solving the problems, state or sketch qualitatively the expected results or trends.
Questions
Q1-1A QBR Questions Before Reading. Research has shown (J. Exp. Psychol. Learn. Mem. Cogn., 40, 106–114 (2014)) that if you ask a question of the material before reading the material you will have greater retention. Consequently, the first question of every chapter will have such a question on that chapter’s material. For Chapter 1, the question is “Is the generation term, G, the only term in the mole balance that varies for each type of reactor?”
Q1-2A Go to Chapter 1 Evaluation on the Web site. Click on i>Clicker Questions (http://www.umich.edu/~elements/6e/01chap/iclicker_ch1_q1.html) and view at least five i>clicker questions. Choose one that could be used as is, or a variation thereof, to be included on the next exam. You also could consider the opposite case: explaining why the question should not be on the next exam. In either case, explain your reasoning.

Leave a Reply